SINGAPORE – Starting November 1, pregnant women in Singapore will be able to receive a vaccine to help protect their infants from a viral respiratory infection. This infection can cause symptoms like cough and fever, and it may lead to serious conditions such as pneumonia.
The Abrysvo vaccine is designed to protect infants, who are very vulnerable to respiratory syncytial virus (RSV). It helps prevent lower respiratory tract diseases caused by this contagious virus for babies up to six months old.
Symptoms of RSV include cough, fever, and body aches. It can lead to pneumonia and bronchiolitis, which is an inflammation of the small airways in the lungs. The vaccine can be given to pregnant women between 32 and 36 weeks of pregnancy, allowing antibodies to be passed to their babies.
Manufactured by Pfizer, Abrysvo was approved by the Health Sciences Authority on July 23. It is the first vaccine of its kind approved for pregnant women in Singapore.
The vaccine will be available at private general practitioner clinics and hospitals, including KK Women’s and Children’s Hospital. When asked about the cost, KKH stated it would be “made affordable” but did not provide a specific price.
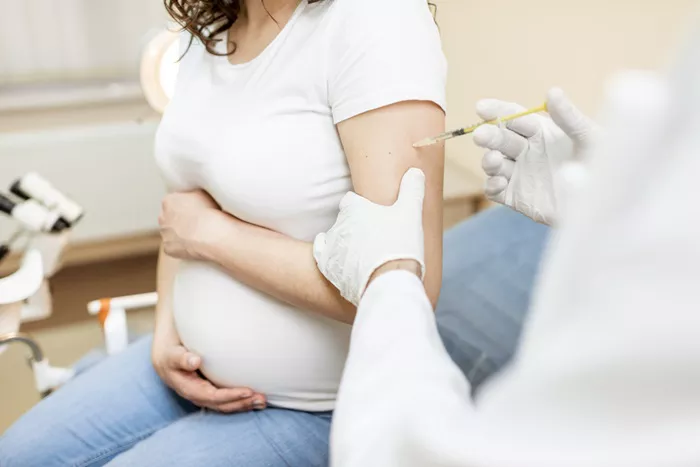
Abrysvo is administered as a single injection into the muscle of the arm. Experts recommend that expectant mothers get the vaccine because infants are particularly vulnerable to RSV.
Dr. Mohana Rajakulendran, a consultant pediatrician at Petite Practice in Joo Chiat, noted that getting RSV during infancy could lead to long-term issues like asthma and wheezing.
Professor Anne Goh, a senior consultant at KKH, added that RSV is a common cause of bronchiolitis and pneumonia in young infants, often resulting in hospitalization. She emphasized that if fewer babies contract RSV, they will have milder illnesses and won’t require hospital visits.
While the vaccine primarily protects newborns, it also offers some protection to pregnant women against RSV.
RSV is the leading cause of acute lower respiratory tract infections in children under five worldwide. According to a study published in the Lancet, it leads to three million hospitalizations and 60,000 deaths each year. In Singapore, RSV is responsible for over 3% of hospitalizations among infants under six months, according to research from the U.S. Centers for Disease Control and Prevention.
Dr. Mohana mentioned that some infants born to vaccinated mothers showed slightly lower birth weights and a small increased risk of jaundice. However, all babies remained healthy after delivery without major complications. Jaundice is a common condition in newborns, characterized by a yellowing of the skin and eyes.
Professor Tan Hak Koon, chairman of the obstetrics and gynecology division at KKH, said that side effects for vaccinated mothers are usually mild. They can include headaches, muscle aches, and soreness, redness, or swelling at the injection site.
However, he cautioned that those with a history of severe allergic reactions should avoid the vaccine. Severe reactions can include anaphylaxis, which is a life-threatening condition that can lead to respiratory failure. People allergic to any ingredients in the vaccine should also not take it.
Dr. Mohana advised mothers with a history of preterm deliveries, high blood pressure, or pre-eclampsia to be cautious when considering the vaccine. Those with minor illnesses, like a cold, can still get vaccinated, but those with more serious illnesses should wait until they are healthier.
Doctors recommend that expectant mothers consult with their obstetricians before getting the vaccine.
During clinical trials, Abrysvo showed 91.1% effectiveness in preventing severe lower respiratory tract disease from RSV in infants during the first 90 days after birth when administered to mothers between 32 and 36 weeks pregnant. Its effectiveness was 76.5% in the first 180 days after birth, according to Pfizer.
Abrysvo has also been approved for individuals aged 60 and older. It is the second RSV vaccine available in Singapore for this age group; the GSK vaccine, Arexvy, was the first to be approved in May 2024.
The Health Ministry stated that the vaccine is not part of the immunization schedule. It will continue to monitor data on the vaccine’s effectiveness and any potential rare serious side effects over time.
Related Topics: